
RWE Design in the Age of Data

CODEF Libraries can change our approach to Real World Evidence Generation.
Click below to find out more.
A regulatory requirement for
RWE Design in the Age of Data

FDA Guidance for Real World Data in September 2021 and December 2023 directed researchers regarding factors affecting their assessment of the reliability and relevance of real-world data, and outlined their expectation on how these should be addressed in study protocols.

The guidance specifically recommends the need for Conceptual Definitions and Operational Definitions (CODEF) to be outlined for study design elements (e.g. inclusion/exclusion criteria for study population, exposure, outcomes, covariates).

The guidance specifically recommends the need for Conceptual Definitions and Operational Definitions (CODEF) to be outlined for study design elements (e.g. inclusion/exclusion criteria for study population, exposure, outcomes, covariates).
Drive Efficiency with Pre-curated CODEF Libraries.
Build strong organization foundations for RWE Design in the age of Data for all stakeholders. Access all relevant study design elements and their associated code-lists and value sets at the click of a button, through our pre-curated CODEF Libraries aligned to the research areas relevant to you.
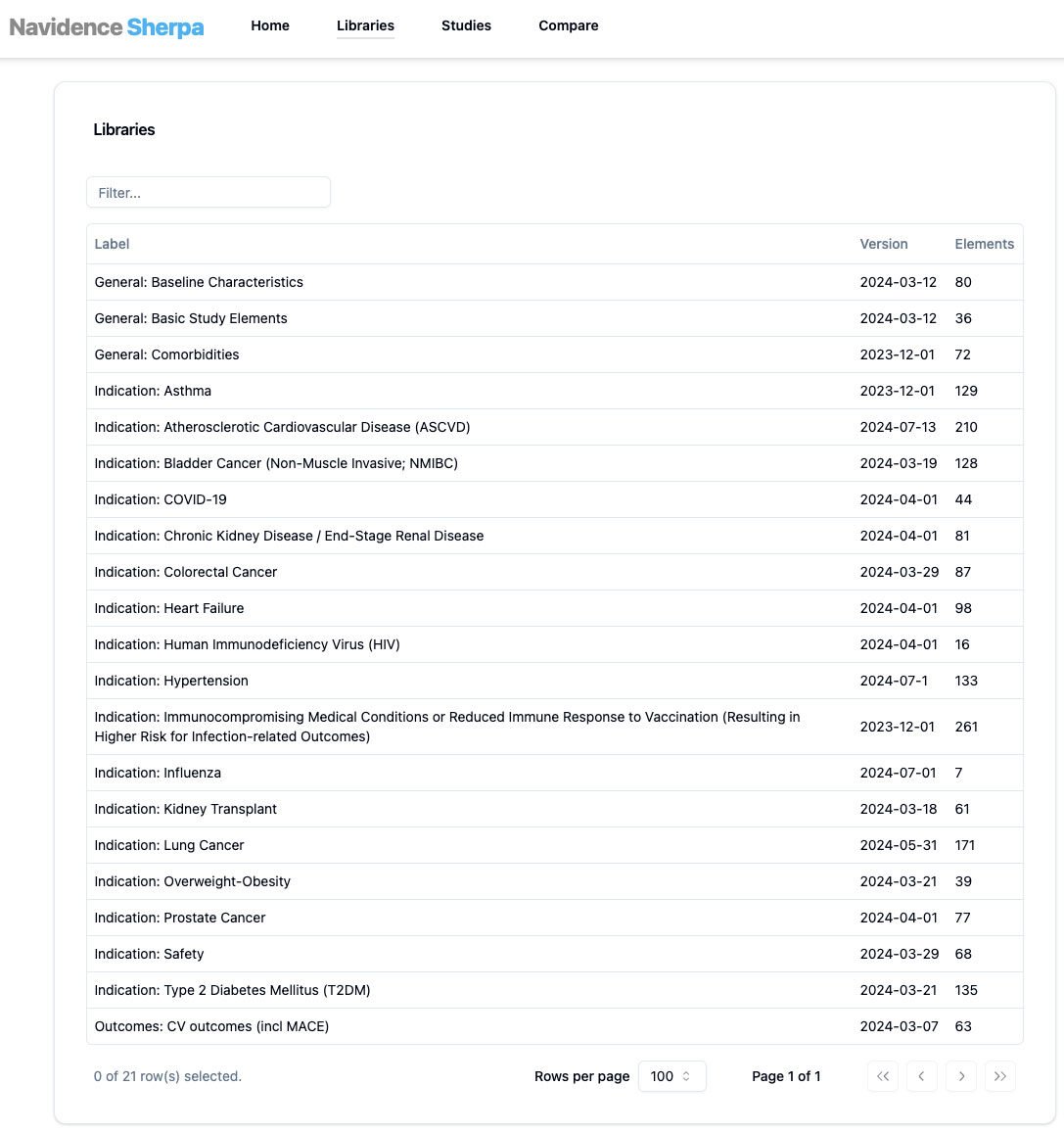
Computable Operational Definitions (CODEF)

"Computable Phenotypes" - Understanding Their Importance in Regulatory Submissions of RWE.
Our CEO Aaron Kamauu, spoke at ISPOR 2024 alongside Marie Bradley from the FDA and Scott DuVall from US Department for Veterans Affairs, to showcase how the Power of CODefs can be used to illustrate fit-for-purpose use of real world data sources to regulators.
Presented at ISPOR Annual Meeting, Atlanta. 4th-8th May 2024.

Objective Fit-4-Purpose Assessment of Real-World Data for Evidence Generation in Type 2
Diabetes Mellitus: A Trial Tokenization Approach
The Navidence Team working alongside our colleagues from Datavant showed how the use of CODefs can derive significant value in the assessment of the value of RWD Tokenisation, as well and providing robust evidence of quality and fit-for-purpose to regulatory agencies.
Presented at ISPOR Annual Meeting, Atlanta. 4th-8th May 2024.

Development of Computable Operational Definitions to Maximize Comparability & Consistency Across a Multi-Data Source Global Real-World Effectiveness Program
The Navidence Team working alongside a team from AstraZeneca to develop source-agnostic Computable Operational Definitions (CODef) to support a multinational, multi-data source real-world effectiveness program.
Presented at the 39th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE) | August 23–27, 2023, Halifax, Nova Scotia, Canada




